Department of Biochemistry & Molecular Biology
dooh@utmb.edu
UTMB Research Experts
Education and Training
Ph.D. Biophysics, Purdue University, USA, 2010
M.S. Physics, Purdue University, USA, 2005
B.S. Physics, Sungkyunkwan University, Korea, 2002
Research Fellow, Mechanobiology Institute, National University of Singapore (Affiliation: University of California Berkeley), 2015 -2020
Research Fellow, HHMI Summer Research, Marine Biological Laboratory in Wood Hole, 2015
Research Fellow, University of Connecticut Health Center, 2010 – 2015
Research Areas
Receptor tyrosine kinase signalling in cancer and mechanobiology. Receptor in host-pathogen interaction
Approaches
Time-lapse live cell imaging, Single-molecule imaging, Single-particle tracking photoactivated localization microscopy (sptPALM), Substrate micropatterning, Physics of protein motion in cell membrane.
Research Interests
Competition between RTKs
Cell senses a variety of extracellular growth factors and signaling molecules through numerous distinct receptor tyrosine kinases (RTKs) on the cell surface. In many cases, the same intracellular signaling
molecule interacts with more than onetype
of RTK. How signals from different RTKs retain the identity of the triggering receptor and how different receptors may synergize or compete remains largely unknown. To approach this issue, our lab utilizes an experimental strategy, combining microscale
patterning and single molecule imaging, to measure the communication between different RTKs signaling for the shared downstream signaling molecules. For example, the competition between ephrin-A1:EphA2 and EGF:EGFR ligand-receptor complexes for Grb2 and
SOS is directly quantified on the same cell surface. Our recent data revealed a type of negative crosstalk interaction fundamentally controlled by chemical mass action and protein copy number limitations. This study and further details may shed light
on the molecular mechanism of achieved tyrosine kinase inhibitor (TKI) resistance in cancer treatment.
(See below: figure and movie).
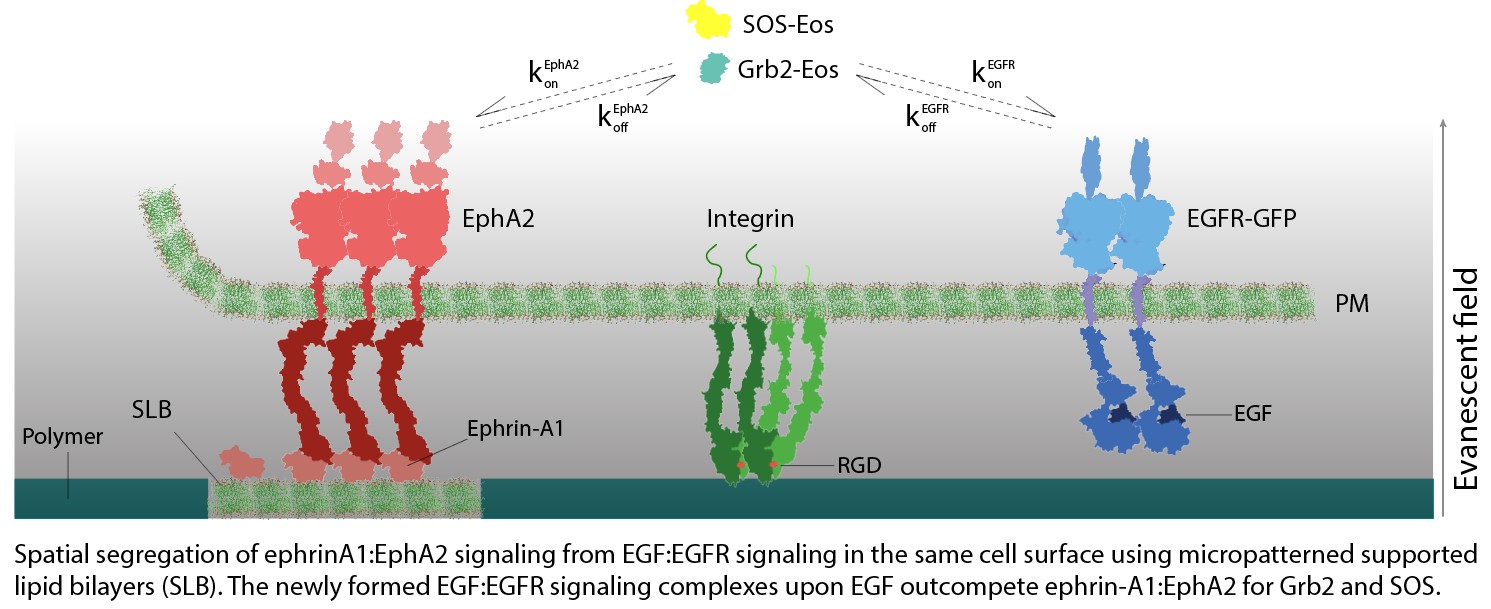
Crosstalk between ephrin/EphA2 with integrin-mediated FA signaling
The EphA2 plays a key role in the assembly of cells into healthy tissues, and dysregulation of this pathway is causally linked to cancer. The molecular mechanisms underlying these functions remain unclear. One key missing aspect to resolve the current controversy in literature is spatial effects of receptor distribution on the cell surface. In vivo, EphA2 activation by ephrin-A1 ligand occurs in spatially 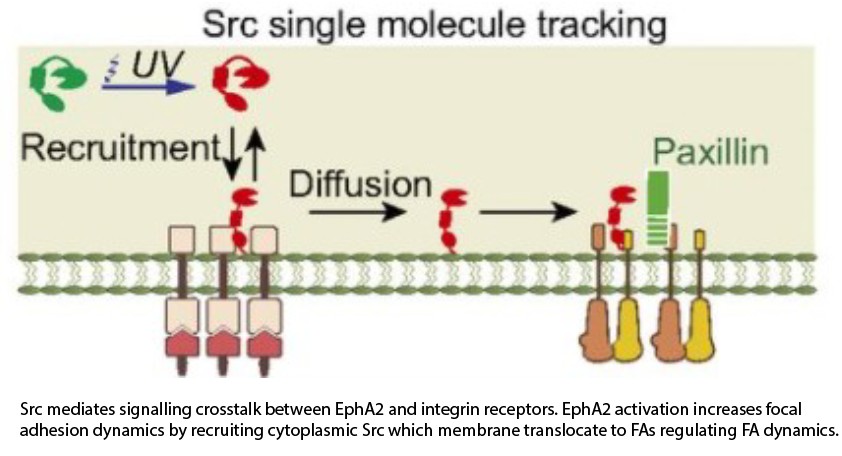 defined regions, but it is not generally possible to reproduce this spatial juxtaposition of signals in classical cell experiments. We used a hybrid substrate with micropatterned supported lipid bilayers (SLB) where ephrin-A1 ligands are present on SLB surface allowing fluid rearrangement, clustering, and activation of EphA2 receptors on the membrane regions. We found that EphA2 activation by ephrin-A1 triggers a local increase in contractility and a global increase in focal adhesion dynamics, a phenotype in agreement with cancer migration. In addition, combining surface patterning and single molecule tracking experiments, we directly observed translocation of Src from sites of EphA2 activation to focal adhesions, revealing a mechanism by which EphA2 modulates focal adhesion dynamics over long distances. Finally, we demonstrate that the EphA2 signalling system is intrinsically sensitive to the spatial organization of activating ligands with polarized ligand presentation leading to directional cell migration.(see figure and movie below).
defined regions, but it is not generally possible to reproduce this spatial juxtaposition of signals in classical cell experiments. We used a hybrid substrate with micropatterned supported lipid bilayers (SLB) where ephrin-A1 ligands are present on SLB surface allowing fluid rearrangement, clustering, and activation of EphA2 receptors on the membrane regions. We found that EphA2 activation by ephrin-A1 triggers a local increase in contractility and a global increase in focal adhesion dynamics, a phenotype in agreement with cancer migration. In addition, combining surface patterning and single molecule tracking experiments, we directly observed translocation of Src from sites of EphA2 activation to focal adhesions, revealing a mechanism by which EphA2 modulates focal adhesion dynamics over long distances. Finally, we demonstrate that the EphA2 signalling system is intrinsically sensitive to the spatial organization of activating ligands with polarized ligand presentation leading to directional cell migration.(see figure and movie below).
Ligand mobility dependent RTK signal amplification
Cell membrane receptors transduce extracellular chemical and physical signals into intracellular signaling systems. Ligand binding to the receptor open entails clustering of the
ligand:receptor complex on the cell membrane, which often appears to have functional consequences. These processes remain poorly understood, however, largely due to a lack of 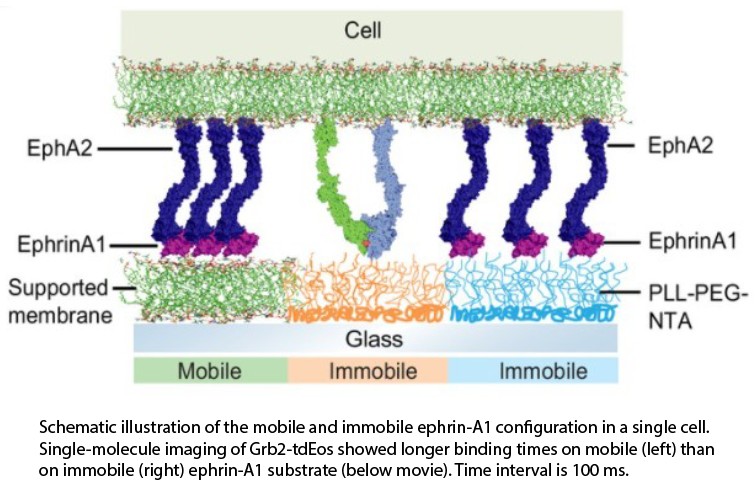 appropriate experimental platforms.
We develop a microfabrication strategy to produce substrates displaying mobile and immobile ligands for a side-by-side comparison of clustered and non-clustered receptors within single cells, using live cell and single molecule imaging. We apply this
approach to characterize ephrin-A1:EphA2 signaling systems and find that EphA2 clustering enhances receptor phosphorylation and subsequent Grb2 adaptor protein interactions relative to non-clustered receptors within the same cell. This type of intracellular
comparison enables a substantially higher degree of quantitative analysis than is possible when comparisons must be made between different cells. This strategy can be readily applied to study other cell receptors.
appropriate experimental platforms.
We develop a microfabrication strategy to produce substrates displaying mobile and immobile ligands for a side-by-side comparison of clustered and non-clustered receptors within single cells, using live cell and single molecule imaging. We apply this
approach to characterize ephrin-A1:EphA2 signaling systems and find that EphA2 clustering enhances receptor phosphorylation and subsequent Grb2 adaptor protein interactions relative to non-clustered receptors within the same cell. This type of intracellular
comparison enables a substantially higher degree of quantitative analysis than is possible when comparisons must be made between different cells. This strategy can be readily applied to study other cell receptors.
(see figure and movie below).
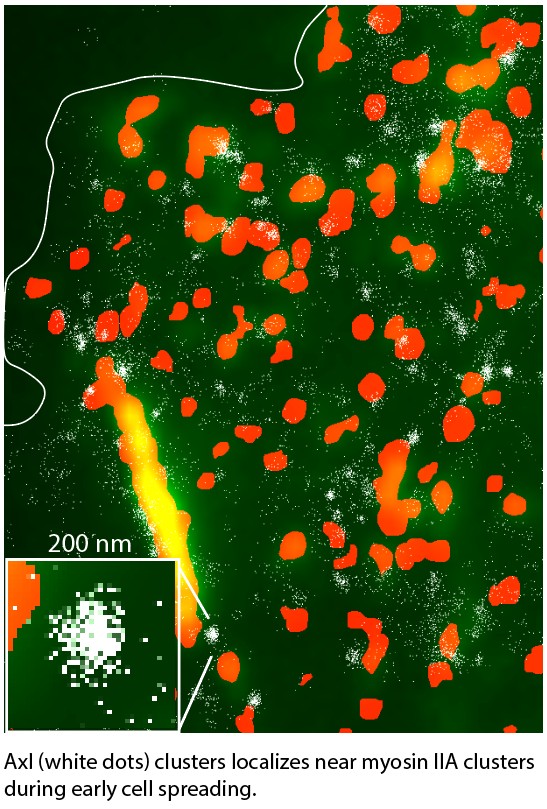
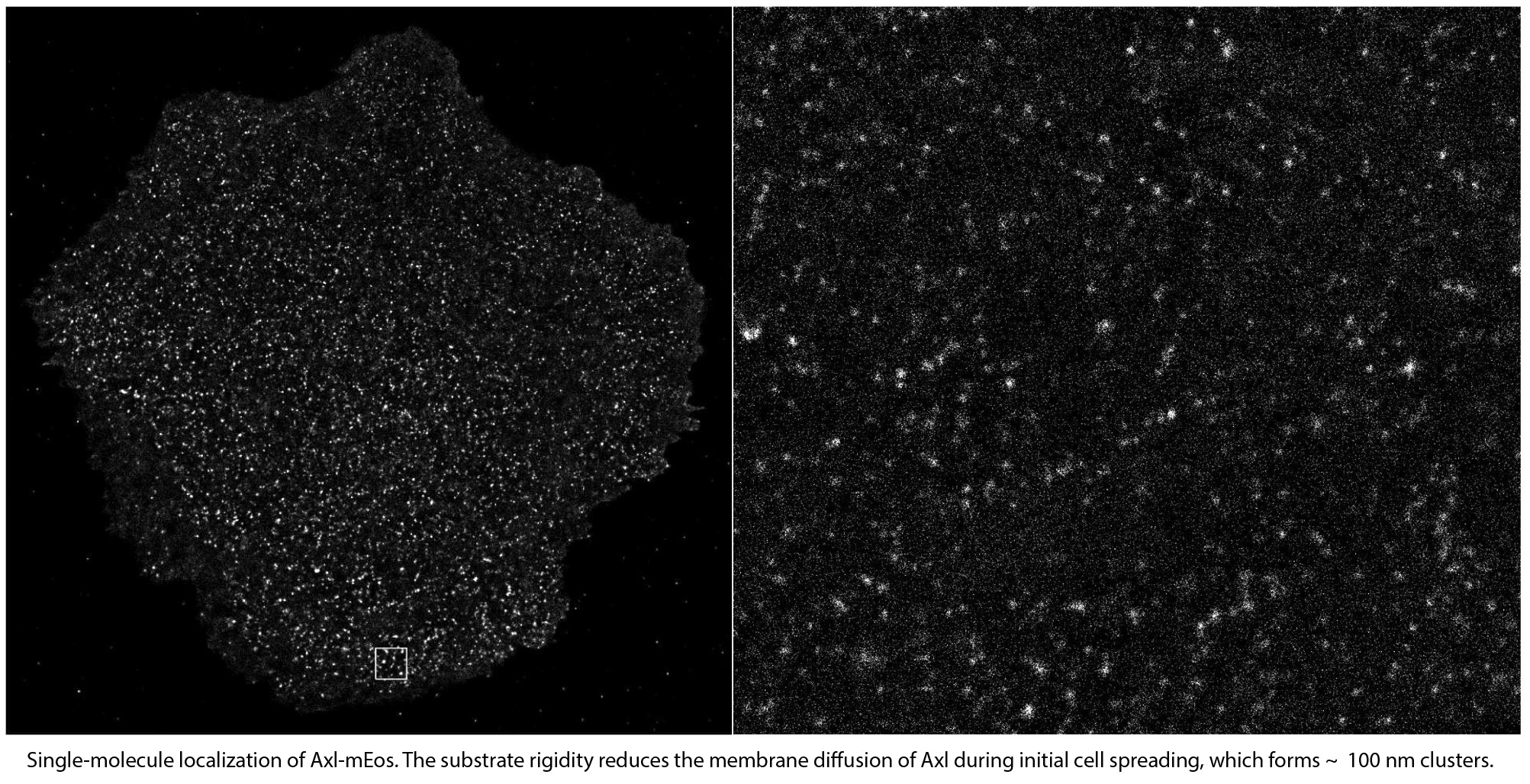
Crosstalk between Axl with myosin IIA
 Substrate rigidity is an important mechanical parameter that leads to cell growth, survival, and apoptosis by linking mechanical-mediated signalling and other receptor signaling. For example, it was
observed decades ago that cells on the rigid substrate activate RTK in serum free media. However, the underlying mechanism is still unknown. Among the receptor tyrosine kinases, Axl is better known to crosstalk with integrin-mediated mechanical signaling,
including rigidity sensing process. We are currently studying how myosin IIA, one of key rigidity sensing components, regulates Axl activation on rigid substrates. Our preliminary data showed that Axl membrane dynamics and function depended on the
cell spreading process controlled by myosin IIA clusters and fibers dynamics. During initial isotropic cell spreading, myosin IIA clusters recruited Axl and Axl formed functional clusters. These Axl clusters were of similar size to those formed
through Gas6 ligand stimulation and triggered the MAPK/Erk upstream pathway, indicating myosin IIA-mediated Axl activation. This phenotype was significantly reduced in polarized initial spreading or overnight adherent cells. Therefore, the pathway
of long-term RTK activation by extracellular matrix stiffness needs to be studied.
Substrate rigidity is an important mechanical parameter that leads to cell growth, survival, and apoptosis by linking mechanical-mediated signalling and other receptor signaling. For example, it was
observed decades ago that cells on the rigid substrate activate RTK in serum free media. However, the underlying mechanism is still unknown. Among the receptor tyrosine kinases, Axl is better known to crosstalk with integrin-mediated mechanical signaling,
including rigidity sensing process. We are currently studying how myosin IIA, one of key rigidity sensing components, regulates Axl activation on rigid substrates. Our preliminary data showed that Axl membrane dynamics and function depended on the
cell spreading process controlled by myosin IIA clusters and fibers dynamics. During initial isotropic cell spreading, myosin IIA clusters recruited Axl and Axl formed functional clusters. These Axl clusters were of similar size to those formed
through Gas6 ligand stimulation and triggered the MAPK/Erk upstream pathway, indicating myosin IIA-mediated Axl activation. This phenotype was significantly reduced in polarized initial spreading or overnight adherent cells. Therefore, the pathway
of long-term RTK activation by extracellular matrix stiffness needs to be studied.
(see figure and movie below).
Therefore, the pathway of long-term RTK activation by extracellular matrix stiffness needs to be studied. The second movie shows photoconverting of Axl-mEos via UV light.
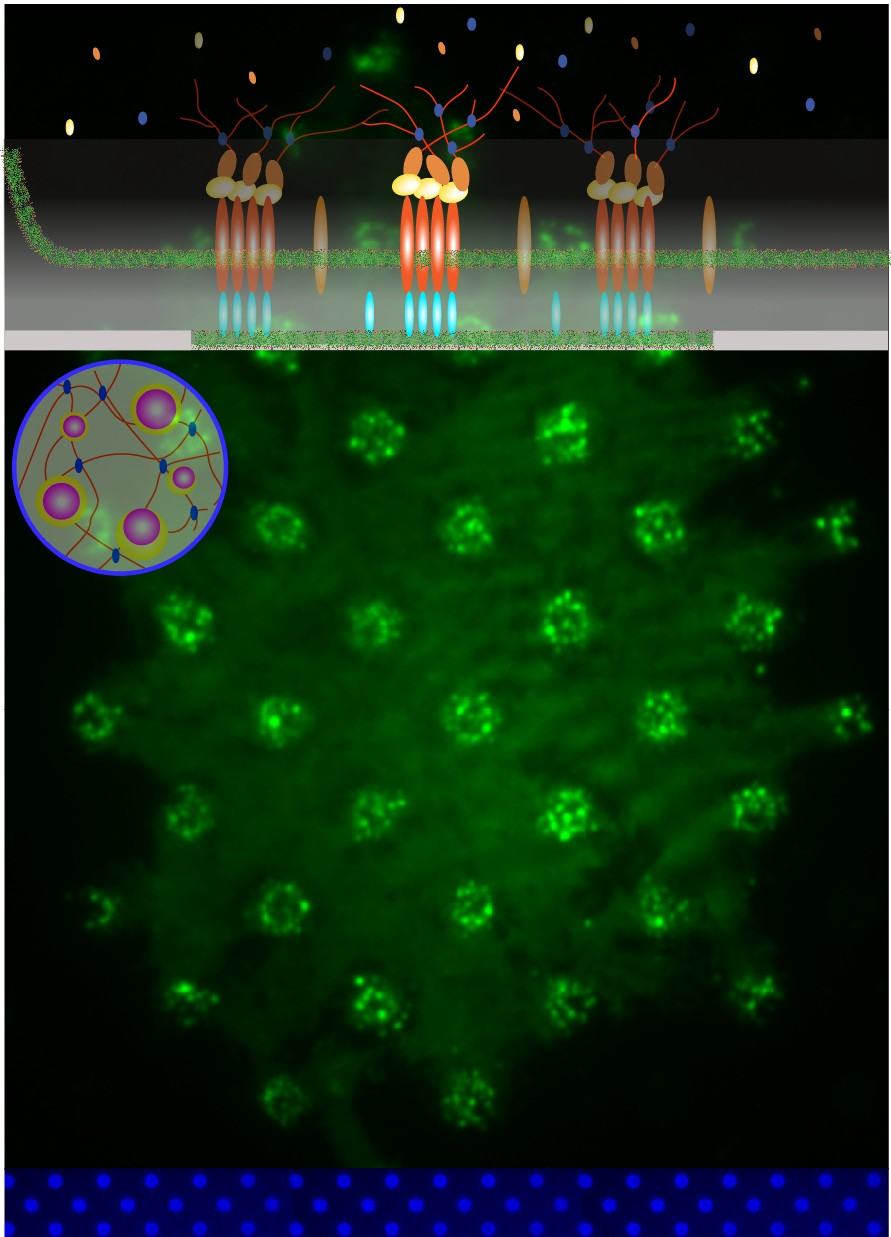
Protein nanocondensates
We have reconstituted elements of the EPEC actin pedestals using supported lipid bilayer patterns with intimin and cells expressing functional Tir. Single-particle superresolution imaging (sptPALM) revealed dynamic functional (pTyr-Tir) Tir nanoclusters (180 nm) that recruit Nck and NWASP. These protein nanoclusters are kinetically sustained through membrane and cytoplasmic exchanges and seed interconnected actin filament networks. Reference
Epilog
Recently, RTK inhibitors combination therapy has been shown to be of great efficacy in clinical and preclinical cancer studies. Targeting specific RTK with different inhibitors can overcome certain types of TKI resistance cells achieved. Direct regulation of one RTK by a different RTK or bypass mechanism has recently been proposed. However, the underlying mechanism needs to be elusive. We hope that our studies on RTKs will help explain the observed clinical data.
Related Publications
- Dongmyung Oh, Xuyao Liu, Michael P. Sheetz, Linda J. Kenney, Small, Dynamic Clusters of Tir-Intimin Seed Actin Polymerization. Nano Micro Small, August 2023
https://doi.org/10.1002/smll.202302580
- Dongmyung Oh*, Zhongwen Chen*, Kabir H Biswas, Funing Bai, Hui Ting Ong, Michael P Sheetz, Jay T Groves, Competition for shared downstream signaling molecules establishes indirect negative feedback between EGFR and EphA2, Biophysical Journal,
121, 2022
https://www.sciencedirect.com/science/article/pii/S0006349522003149
- Zhongwen Chen*, Dongmyung Oh*, Kabir Hassan Biswas, Ronen Zaidel-Bar, Jay T Groves, Probing the effect of clustering on EphA2 receptor signaling efficiency by subcellular control of ligand-receptor mobility, eLife, 10, 2021
https://elifesciences.org/articles/67379
- Zhongwen Chen, Yuhong Cao, Chun-Wei Lin, Steven Alvarez, Dongmyung Oh, Peidong Yang, Jay T Groves, Nanopore-mediated protein delivery enabling three-color single-molecule tracking in living cells, PNAS, 118, 2021
https://www.pnas.org/doi/10.1073/pnas.2012229118
- Zhongwen Chen*, Dongmyung Oh*, Kabir H Biswas, Cheng-Han Yu, Ronen Zaidel-Bar, Jay T Groves, Spatially modulated ephrinA1: EphA2 signaling increases local contractility and global focal adhesion dynamics to promote cell motility, PNAS, 115, 2018
https://www.pnas.org/doi/abs/10.1073/pnas.1719961115
- Dongmyung Oh, Yang Yu, Hochan Lee, Jae-Hyung Jeon, Barry L Wanner, Ken Ritchie, Asymmetric polar localization dynamics of the serine chemoreceptor protein Tsr in Escherichia coli, PLOS one, 2018
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0195887
- Joshua A Jadwin*, Dongmyung Oh*, Timothy G Curran, Mari Ogiue-Ikeda, Lin Jia, Forest M White, Kazuya Machida, Ji Yu, Bruce J Mayer, Time-resolved multimodal analysis of Src Homology 2 (SH2) domain binding in signaling by receptor tyrosine kinases,
eLife, 5, 2016
https://elifesciences.org/articles/11835